
Our professional team will support you to go further!
Get a Quote. Ask a Question. We respond within 10 to 15 minutes during business hours.
tablet supplements manufacturer
Searching for a high-capacity, high-quality, and highly certified tablet manufacturer to develop and produce exceptional nutritional or dietary supplements for your customers? Look no further than Gensei.
We understand that choosing an affordable yet high-quality tablet supplement manufacturer can be challenging. That’s why we’ve put together a comprehensive guide to help you make an informed decision.
In this article, we’ll walk you through the tablet supplement manufacturing process, introduce you to our wide range of products and services, and answer your questions about custom tablet supplement manufacturing .
what is tablet supplement manufacturing?

Tablet supplement manufacturing is the process of converting powdered ingredients (active nutrients and excipients) into solid, precisely dosed, and stable tablet forms through compression. It’s a highly common method for producing dietary supplements due to its cost-effectiveness, ease of use for consumers, and ability to incorporate a wide range of ingredients.
- This initial stage involves combining the active ingredients (vitamins, minerals, herbs, amino acids, etc.) with excipients (inactive ingredients).
- Excipients play crucial roles:
- Binders: To hold the tablet together (e.g., microcrystalline cellulose, starch, gums).
- Fillers/Diluents: To add bulk for a reasonable tablet size (e.g., lactose, dicalcium phosphate).
- Disintegrants: To help the tablet break apart in the body for nutrient release (e.g., croscarmellose sodium).
- Lubricants: To prevent the powder from sticking to the tablet press machinery (e.g., magnesium stearate).
- Glidants: To improve powder flow (e.g., colloidal silicon dioxide).
- Colors/Flavors: For aesthetic appeal or to mask taste.
Ingredient Sourcing & Quality Control:
- All raw materials undergo rigorous testing for identity, purity, potency, and contaminants (e.g., heavy metals, microbes) before being approved for use.
Blending & Mixing:
- The precisely weighed active ingredients and excipients are thoroughly blended in large industrial mixers to ensure a homogeneous distribution of all components throughout the batch. This is critical for consistent dosage in every tablet.
Granulation (Optional but Common):
- This step transforms fine powders into larger, denser, and more free-flowing granules. It’s often necessary if the raw powders don’t compress well directly or if they have poor flow properties, which can lead to inconsistencies.
- Wet Granulation: Involves adding a liquid binder solution to the powder mix, creating a wet mass, which is then dried and sieved into granules.
- Dry Granulation: Involves compacting the powders into larger pieces (slugs or roller compaction) and then milling them into granules, without the use of liquids.
- Direct Compression: If ingredients have good flow and compressibility properties inherently, this granulation step can be skipped, making it the simplest and most cost-effective method.
Tablet Compression:
- The blended powder or granules are fed into a tablet press machine.
- The powder fills a die cavity, and then an upper punch and a lower punch apply immense pressure to compact the material into a solid tablet of a specific size, shape, and hardness.
- Modern tablet presses are highly automated, ensuring consistent weight, thickness, and hardness for each tablet. They can also create specific imprints (like logos or scores for breaking).
Tablet Coating (Optional):
- Many tablets undergo a coating process for various reasons:
- Improved Swallowing: Makes them easier to swallow.
- Masking Taste/Odor: Covers unpleasant flavors or smells.
- Protection: Shields ingredients from moisture, light, or oxygen, extending shelf life.
- Delayed/Enteric Release: Prevents dissolution in the stomach, allowing the tablet to release its contents in the intestine.
- Aesthetics: Adds color and a smooth finish.
- Many tablets undergo a coating process for various reasons:
Final Quality Control & Testing:
- Finished tablets undergo a series of tests to ensure they meet specifications:
- Weight Uniformity: To confirm consistent dosage.
- Hardness: To ensure resistance to breakage during handling.
- Friability: To test resistance to crumbling.
- Disintegration Time: How long it takes for the tablet to break apart in a liquid.
- Dissolution Rate: How quickly the active ingredients dissolve and become available.
- Potency, Purity & Contaminants: Final checks on active ingredient levels, absence of impurities, and microbial safety.
- Finished tablets undergo a series of tests to ensure they meet specifications:
Packaging & Labeling:
- Once all quality checks are passed, tablets are counted and filled into primary packaging (bottles, blister packs) and then labeled according to regulatory guidelines.
Features of working with tablet supplements manufacturer
Partnering with a tablet supplement manufacturer, such as Gensei, offers significant advantages for businesses aiming to develop and market high-quality dietary supplements in tablet form. Tablets are a popular delivery format due to their durability, precise dosing, and versatility. Below are the key features of working with a reputable tablet supplement manufacturer, tailored to highlight the value Gensei brings to your brand:
- Customized Formulations:
- Manufacturers collaborate to create tailored tablet formulations, blending active ingredients like vitamins, minerals, or botanicals (e.g., turmeric, ashwagandha) to meet specific health goals or market demands.
- Gensei’s expertise in complex blends, including high-potency or time-release formulas, ensures your brand delivers effective, differentiated products.
- Flexible Tablet Design:
- Options for tablet size, shape (e.g., round, oval, caplet), and coatings (e.g., enteric, film-coated, or chewable) to enhance consumer appeal and functionality.
- Gensei customizes tablet appearance and texture, aligning with your brand’s identity and target audience preferences.
- Private Labeling and Branding:
- Comprehensive private label services allow your brand to market tablets with custom logos, labels, and packaging (e.g., bottles, blisters, bulk).
- Gensei’s branding support creates visually appealing, market-ready packaging that strengthens your brand’s presence and consumer trust.
- High-Quality Production Standards:
- Adherence to Good Manufacturing Practices (GMP) and regulatory standards (e.g., FDA, Health Canada, ISO) ensures safe, consistent, and high-quality tablets.
- Gensei’s rigorous testing for potency, purity, disintegration, and contaminants (e.g., heavy metals, microbes) guarantees reliable products that elevate your brand’s reputation.
- Advanced Manufacturing Capabilities:
- State-of-the-art compression and coating technologies produce tablets with precise dosing, uniform weight, and optimal hardness for durability and shelf stability.
- Gensei’s facilities support specialized formats like bilayer tablets, chewables, or effervescent tablets, offering versatility for your brand’s product line.
- Scalable Production Capacity:
- Flexible production runs accommodate small batches for startups or large-scale orders for established brands, ensuring scalability as your brand grows.
- Gensei’s high-capacity lines deliver consistent quality and meet tight market deadlines, keeping your brand competitive.
- End-to-End Support:
- From formulation development and ingredient sourcing to regulatory compliance and packaging, manufacturers provide full-service solutions to streamline the process.
- Gensei’s dedicated team offers expert guidance, ensuring your brand navigates complex regulations and launches products efficiently.
- Cost Efficiency and Budget Control:
- Outsourcing eliminates the need for costly in-house equipment, facilities, or staff, reducing overhead for your brand.
- Gensei’s transparent pricing and efficient processes deliver high-quality tablets within budget, maximizing your brand’s ROI.
- Consumer-Centric Appeal:
- Tablets offer precise dosing, portability, and long shelf life, appealing to consumers who value convenience and reliability.
- Gensei enhances consumer experience with options like flavored chewables, easy-swallow coatings, or sustained-release tablets, driving loyalty for your brand.
- Innovation and Market Trends:
- Manufacturers stay ahead of trends, offering innovative tablet formats like plant-based, organic, or functional supplements (e.g., immunity, joint health).
- Gensei’s R&D expertise helps your brand capitalize on emerging markets, such as clean-label or condition-specific tablets, keeping you competitive.
- Sustainability Options:
- Eco-friendly practices, such as recyclable packaging or minimal-waste production, appeal to environmentally conscious consumers.
- Gensei offers sustainable solutions, like plant-based excipients or biodegradable bottles, aligning your brand with green trends.
- Fast Turnaround and Reliability:
- Efficient production timelines and robust supply chains ensure quick delivery, critical for meeting market demands or seasonal campaigns.
- Gensei’s reliable processes and proactive communication keep your brand on track from concept to market.
Common Tablet Shapes & Typical Dimensions/Weights
The weight of a tablet for a given size will heavily depend on the density of the powdered blend. A denser powder will allow more active ingredient to fit into a smaller tablet size.
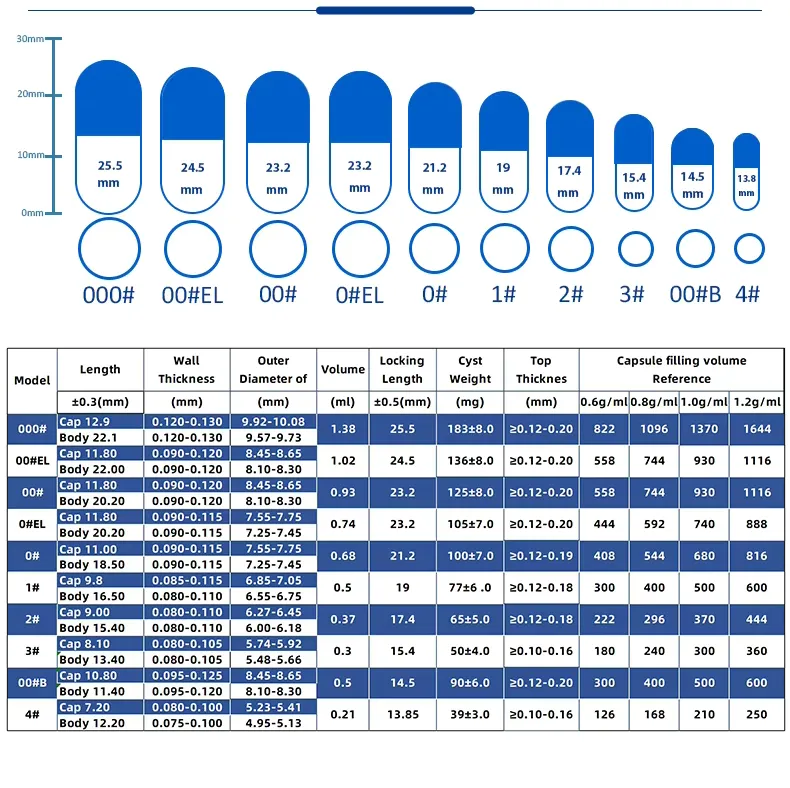
| Shape | Typical Diameter (Round) or Length (Oblong/Oval) | Typical Width (Oblong/Oval) | Common Weight Range (mg) | Notes |
|---|---|---|---|---|
| Round | 6 mm to 13 mm | N/A | 100 mg - 1000 mg+ | Very common, easy to swallow for many. Can be scored for splitting. |
| Oval | 12 mm to 19 mm | 6 mm to 10 mm | 250 mg - 1000 mg+ | Elongated shape, often perceived as easier to swallow than large rounds. |
| Oblong | 18 mm to 22 mm+ | 7 mm to 10 mm | 500 mg - 1500 mg+ | Longer and narrower than ovals, good for higher dosages while maintaining swallowability. Often referred to as "caplets" if coated. |
| Caplet | (Typically Oblong or Oval) | (Typically Oblong or Oval) | Varies, often higher | A portmanteau of "capsule" and "tablet," indicating a smooth, often coated, tablet in an elongated capsule-like shape. Designed for ease of swallowing. |
| Mini-Tablet | 2 mm to 5 mm | (Typically Round) | 20 mg - 150 mg | Used for precise, small doses, combination products (multiple mini-tablets in a capsule), or for patient populations with swallowing difficulties. |
| Custom | Varies widely | Varies widely | Varies widely | Manufacturers can create unique shapes (e.g., hearts, stars, specific brand logos) with custom tooling, allowing for strong brand differentiation. Size will depend on the dose and design. |
Choose the Right Package for Your tablet supplements
Bottles (Plastic or Glass)
- Description: The most widely used packaging for multi-dose tablet supplements.
- Plastic Bottles: Commonly made from HDPE (High-Density Polyethylene) or PET (Polyethylene Terephthalate). HDPE offers excellent moisture barriers, while PET provides good clarity for product visibility. PP (Polypropylene) is also used.
- Glass Bottles: Offer superior barrier properties against moisture, oxygen, and odors, and are often chosen for premium or sensitive products. Amber or cobalt blue glass provides excellent UV protection.
- Pros:
- Excellent Protection: Provides strong physical protection against crushing and external contamination. Good moisture and oxygen barriers, especially with appropriate cap liners and desiccants.
- Child-Resistant Closures (CRCs): Readily available push-and-turn or squeeze-and-turn caps are essential for safety, especially for supplements that might appeal to children.
- Branding Space: Offers ample surface area for attractive labels, branding, and all necessary regulatory information.
- Consumer Familiarity: A widely accepted and convenient format for daily use products.
- Resealable: Allows consumers to maintain freshness after opening.
- Cons:
- Bulk & Weight: Can be heavier (especially glass) and bulkier, increasing shipping costs and shelf space requirements.
- Plastic Concerns: While recyclable, concerns about plastic waste persist.
- Description: The most widely used packaging for multi-dose tablet supplements.
Blister Packs
- Description: Individual tablets are sealed within separate cavities formed from plastic (e.g., PVC, PVDC for barrier properties) with a foil backing. Often presented in a paperboard carton.
- Pros:
- Superior Individual Protection: Each tablet is hermetically sealed, providing excellent protection against moisture, oxygen, and contamination until the moment of consumption.
- Portion Control & Hygiene: Ensures precise dosage and maintains the freshness and integrity of each individual tablet. Great for travel.
- Tamper Evidence: Clear visual indication if a tablet has been accessed.
- Compliance Packaging: Can be designed with calendarized dosing (e.g., day-of-the-week printing) to help consumers track their intake.
- Enhanced Presentation: Often gives a more “pharmaceutical” or premium feel.
- Cons:
- Higher Cost per Unit: Generally more expensive per tablet compared to bottles, especially for high counts.
- Limited Branding Space: The primary branding surface is usually on the outer carton rather than the blister itself.
- Accessibility: Can be challenging for some individuals (e.g., those with arthritis or dexterity issues) to open.
- Environmental Impact: Combining plastic and foil can make recycling more complex, though efforts for mono-material blisters are ongoing.
Pouches / Bags (Flexible Packaging)
- Description: Typically multi-layer stand-up pouches with re-sealable zippers, made from barrier films (e.g., PET, aluminum foil, PE). Less common for initial retail sale of tablets, but gaining traction for bulk refills or multi-serve powders, which might include tablets. Single-serving sachets/stick packs are also flexible options for a few tablets or powders.
- Pros:
- Lightweight & Portable: Reduces shipping costs and is convenient for consumers.
- Space Efficient: Takes up less storage and shelf space than rigid containers.
- Good Barrier Properties: Can offer excellent protection against moisture and oxygen with the right film laminates.
- Ample Branding Space: Provides a large, flexible canvas for graphics.
- Sustainability Potential: Some recyclable options are emerging.
- Cons:
- Less Physical Protection: Tablets are more susceptible to crushing or chipping than in rigid bottles or blister packs.
- Dispensing: Can be less precise for multi-dose tablets than bottles.
- Child Resistance: More challenging to implement robust child-resistant features compared to bottles.
Key Factors When Choosing Packaging for Tablet Supplements
Product Protection & Stability:
- Moisture Barrier: Crucial for dry tablet forms to prevent degradation, dissolution, or caking.
- Oxygen Barrier: Essential for ingredients sensitive to oxidation (e.g., certain vitamins, probiotics).
- Light Protection: Opaque or amber-colored packaging (bottles, foils) is vital for light-sensitive ingredients.
- Physical Integrity: The packaging must protect tablets from breaking, chipping, or crumbling during transit and handling.
Consumer Experience & Convenience:
- Ease of Use: How easy is it for the consumer to open, dispense, and reseal the product?
- Child Resistance (CR): For many supplement tablets, CR closures are a regulatory requirement and a critical safety feature, especially for products that might be mistaken for candy.
- Portability: Is the product intended for daily home use, or for travel and on-the-go consumption?
- Dose Accuracy: Blister packs excel here, ensuring precise dosage with each use.
Branding & Marketing:
- Visual Appeal: The packaging is a primary marketing tool. Consider material finish, shape, color, and label design to convey your brand’s message (e.g., premium, natural, value).
- Information Space: Ensure enough space for all mandatory regulatory labeling (Supplement Facts, ingredients, warnings, etc.) and compelling marketing copy.
Cost & Sustainability:
- Material & Production Cost: Evaluate the cost of the packaging material, as well as the efficiency and cost of the filling, sealing, and labeling processes.
- Shipping Costs: Lighter, more compact packaging (e.g., pouches or well-designed bottles) can reduce freight expenses.
- Sustainability Goals: Consider using recycled content (PCR plastics), recyclable materials (glass, certain plastics), or options that reduce overall material usage.
Regulatory Compliance:
- Tamper-Evident Features: Mandatory for most dietary supplements (e.g., induction seals, tear strips, film wraps).
- Labeling Requirements: Ensure the chosen packaging format allows for all required information to be displayed clearly and legibly.
What Makes Us Unique As A tablet supplement manufacturer?
What makes a tablet supplement manufacturer unique often comes down to their specialized capabilities, flexibility, and deep understanding of the complexities of dry dosage forms.
- Comprehensive Formulation Expertise: We stand out through our deep understanding of tablet formulation. Our experts meticulously blend active ingredients with excipients, ensuring optimal powder flow, compressibility, and stability. This precise scientific approach guarantees that even the most complex nutrient combinations are consistently and effectively delivered in every single tablet.
- Mastery of All Compression Methods:Our uniqueness lies in our versatility across all tablet compression techniques. Whether your ingredients require the efficiency of direct compression, the enhanced flow and uniformity of wet granulation, or the specialized, moisture-sensitive approach of dry granulation, we possess the precise capabilities and equipment to manufacture your tablets using the most suitable and efficient method for your unique formula.
- Advanced Coating & Release Technologies:Beyond basic film coating, we specialize in advanced tablet coating and release technologies. This includes developing enteric coatings to protect sensitive ingredients from stomach acid, sustained or extended-release coatings to ensure prolonged nutrient delivery, and innovative taste-masking solutions that enhance the consumer experience, offering your product superior functionality and market appeal.
- Extensive Customization & Aesthetic Excellence:We offer unparalleled customization options to make your brand distinctive. From crafting tablets in unique shapes and sizes to ensuring precision imprints (like logos or score lines) and achieving vibrant, consistent coloring, our aesthetic excellence ensures your product not only performs effectively but also looks exceptional on the shelf, reflecting your brand's quality.
- High-Volume, Precision Manufacturing:Our facilities are equipped with state-of-the-art, high-speed tablet presses and automated systems, enabling us to manage high-volume production without compromising on quality. This technological advantage ensures that millions of tablets are manufactured with exceptional precision in weight, hardness, and content uniformity, providing reliable and consistent product batches every time.
- Rigorous Tablet-Specific Quality Control:Our commitment to quality extends through a rigorous, tablet-specific quality control process. Beyond standard potency and purity checks, we meticulously test for critical tablet parameters such as hardness, friability, disintegration time, and dissolution rates. This detailed approach guarantees that your tablets are robust, break down correctly, and release their active ingredients as intended for optimal efficacy.
- Comprehensive Stability & Shelf-Life Expertise:We possess a deep understanding of how various ingredients interact within a dry tablet form, along with factors like moisture migration and oxidation. This expertise, combined with optimal packaging solutions, allows us to formulate and manufacture tablets that maintain their potency, integrity, and appearance throughout their declared shelf life, providing long-term product reliability.
- Proactive Problem-Solving & Technical Support:What truly sets us apart is our proactive approach to problem-solving and dedicated technical support. Our experienced team can expertly identify and resolve common tablet manufacturing challenges—such as sticking, capping, or weight variation—ensuring a smooth production process and consistently high-quality output for your peace of mind.
- Strong Regulatory Compliance:We operate under strict cGMP (Current Good Manufacturing Practices) guidelines, specifically tailored for solid dosage forms. Our robust compliance framework ensures that every tablet produced meets or exceeds global quality, safety, and purity standards, facilitating seamless market entry and upholding the highest level of trust in your brand.
Tablet Supplement Manufacturing Certificate
By getting these certifications, and following rules, we can ensure their products are safe and high quality. The products will also follow the rules.






Frequently Asked Questions About capsule supplement manufacturing
Tablets are highly versatile and can incorporate a wide range of powdered, granular, and crystalline ingredients, including vitamins, minerals, herbal extracts, amino acids, and some specialized compounds.
Excipients are inactive ingredients crucial for tablet formation. They act as binders (to hold the tablet together), fillers (to achieve a suitable size), disintegrants (to help the tablet break down), lubricants (to prevent sticking), and sometimes glidants, colors, or flavors.
Yes, tablet manufacturing is well-suited for high-dose ingredients. The tablet size will be adjusted accordingly, or techniques like dry granulation can increase material density to fit more active compounds into a manageable size.
Ingredients that are sticky, highly hygroscopic (absorb moisture), poorly flowing, or very sensitive to compression force or heat can be challenging. However, advanced granulation techniques and excipient selection often overcome these difficulties.
The main methods are: Direct Compression (simplest, for free-flowing powders), Wet Granulation (improves powder flow and compressibility by adding liquid binder), and Dry Granulation (for moisture-sensitive ingredients, involves compacting and then milling into granules). The choice depends on the raw material properties.
Granulation improves the flowability and compressibility of powders, preventing issues like inconsistent tablet weight, capping (tablet top separating), or lamination (tablet layers separating) during the compression process.
Tablet coating applies a thin layer to the tablet's surface. It's used to: mask unpleasant taste/odor, improve swallowing, protect sensitive ingredients from moisture/light, control the release of active ingredients (e.g., enteric coating), and enhance visual appeal.
Through precise weighing of raw materials, thorough blending to ensure homogeneity, and automated tablet presses that maintain consistent weight and density for every tablet. Final product testing verifies active ingredient potency.
Key tests include: Weight Uniformity (for consistent dosage), Hardness (resistance to breaking), Friability (resistance to crumbling), Disintegration Time (how fast it breaks apart), and Dissolution Rate (how fast the active ingredients dissolve).
Stability testing is conducted under various environmental conditions (temperature, humidity) over time. This data helps predict how long the tablet will remain potent and stable, allowing for an accurate expiration date.
Careful formulation, proper processing (like granulation and controlled drying), tablet coating (especially film coatings), and appropriate packaging with desiccant packets are all used to protect tablets from moisture.
MOQs for tablets vary by manufacturer and product complexity but are generally in the hundreds of thousands to millions of units, reflecting the efficiency of high-speed tablet presses.
Common options include plastic or glass bottles (often with child-resistant caps), blister packs (for individual dose protection and compliance), and sometimes pouches for bulk or refill purposes.
The timeline can vary significantly based on formulation complexity, raw material sourcing, testing requirements, and order size. Generally, it can range from 4 to 8 months for a new product, or less for reorders.
Reputable tablet supplement manufacturers are cGMP (Current Good Manufacturing Practices) compliant, often registered with the FDA, ensuring products are consistently produced and controlled according to strict quality standards.

