Introduction
Sugars do much more than sweeten our food. In biology, they are key players in how cells function, communicate, and stay healthy. Two important sugars, N-Acetylgalactosamine (GalNAc) and N-Acetylglucosamine (GlcNAc), are special types called amino sugars. These molecules have unique structures that make them vital for life. At first glance, they seem similar, but small differences lead to big roles in our bodies and in industries like dietary supplements. As a leading manufacturer of dietary supplement ingredients and OEM products, companies like NutriSynth Solutions use GlcNAc to create high-quality health products. This article compares GalNAc and GlcNAc, exploring their structures, biological roles, and exciting applications in science, medicine, and supplements.
Chemical Structures
To understand GalNAc and GlcNAc, we start with their chemistry. Both are monosaccharides—single sugar units—that belong to the amino sugar family. This means they have a nitrogen-containing group that gives them special properties. Let’s look at their structures.

GalNAc: The Galactose Cousin
GalNAc, or N-Acetylgalactosamine, comes from a sugar called D-galactose. Its chemical formula is C₈H₁₅NO₆. The “N-acetyl” part means that at the second carbon atom (C-2) in its six-membered ring, a hydroxyl group (-OH) is replaced by an acetylated amine group (-NHCOCH₃). This group acts like a chemical tag, making GalNAc recognizable to proteins and enzymes.
CH₂OH | O C6—C5 / \ | | C1–C2—-C4 | | | OH NHAc OH (axial at C-4) | C3 | OH- The ring is a six-membered pyranose structure.
- C-1 has an OH group (typically in β-configuration, pointing up).
- C-2 has an NHAc (acetylated amine, -NHCOCH₃) group.
- C-4 has an axial OH group (pointing up), which is the key difference from GlcNAc.
- C-6 has a CH₂OH group.
- Other OH groups are at C-3 and C-5, typically equatorial.
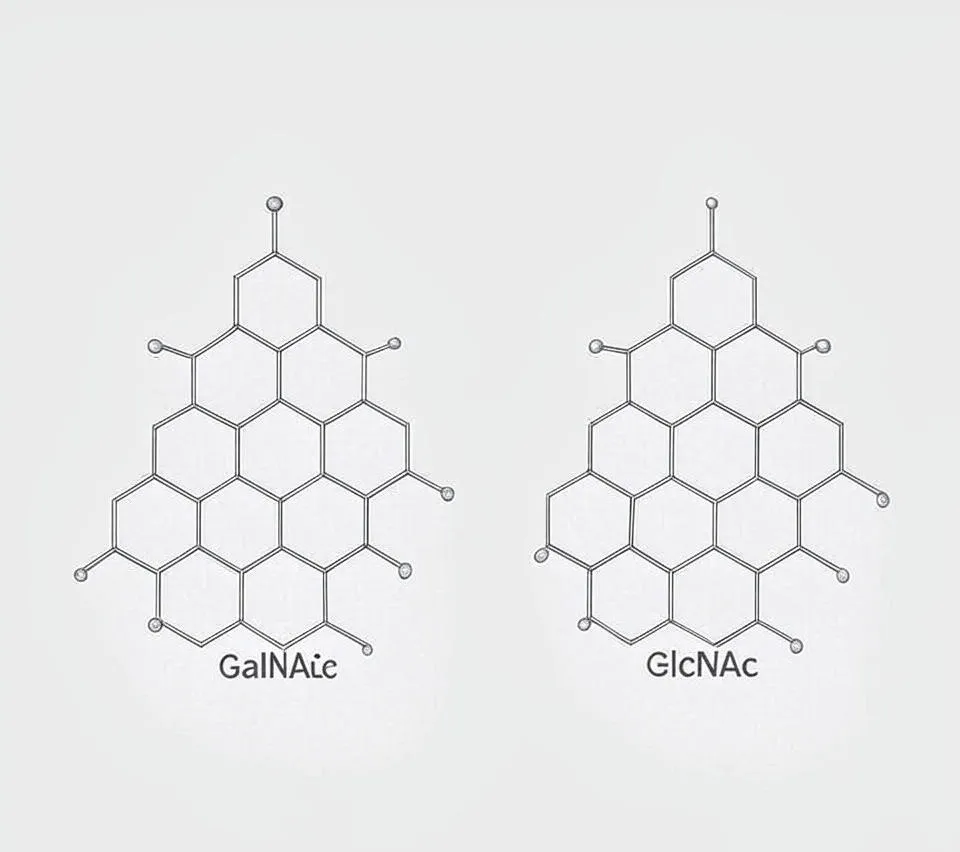
A key feature of GalNAc is the hydroxyl group on its fourth carbon (C-4). This group is in an axial position, meaning it points upward in the sugar’s ring structure. This small detail affects how GalNAc interacts with other molecules. The molecule also has a hydroxymethyl group (-CH₂OH) at C-6 and hydroxyl groups at C-1, C-3, and C-5, which help it connect with other sugars or proteins.
GlcNAc: The Glucose Relative
GlcNAc, or N-Acetylglucosamine, is based on D-glucose, another common sugar. It has the same chemical formula as GalNAc: C₈H₁₅NO₆. Like GalNAc, it has an acetylated amine group at C-2, making it an amino sugar. The key difference is at C-4, where the hydroxyl group is in an equatorial position, lying flat along the ring’s plane. This change makes GlcNAc behave differently in biological systems.
CH₂OH | O C6—C5 / \ | | C1–C2—-C4 | | | OH NHAc OH (equatorial at C-4) | C3 | OH- The ring is a six-membered pyranose structure.
- C-1 has an OH group (typically in β-configuration, pointing up).
- C-2 has an NHAc (acetylated amine, -NHCOCH₃) group.
- C-4 has an equatorial OH group (lying flat), distinguishing it from GalNAc.
- C-6 has a CH₂OH group.
- Other OH groups are at C-3 and C-5, typically equatorial.
GlcNAc also has a hydroxymethyl group at C-6 and hydroxyl groups at C-1, C-3, and C-5, allowing it to form chains or complex structures. Companies like NutriSynth Solutions use GlcNAc as a raw material to produce dietary supplements, leveraging its natural role in the body to support joint health and other benefits.
The Key Difference
GalNAc and GlcNAc are epimers, differing only at C-4. The axial (GalNAc) versus equatorial (GlcNAc) position of the C-4 hydroxyl group changes how these sugars fit into enzymes or bind to proteins. This tiny structural difference is like a molecular signature that shapes their unique roles.
Biological Functions
GalNAc and GlcNAc have distinct jobs in living organisms, largely through a process called glycosylation, where sugars are attached to proteins or lipids (fats) to modify their function. Let’s explore their roles.
GalNAc: The Star of O-Linked Glycosylation
GalNAc is central to O-linked glycosylation. This process attaches sugars to the oxygen atom of serine or threonine amino acids in proteins. GalNAc often serves as the first sugar in these chains, anchoring them to proteins like mucins. Mucins form the slimy mucus layers in our gut, lungs, and eyes, protecting tissues, trapping germs, and keeping surfaces moist.
GalNAc also helps define blood group antigens. For example, in people with blood type A, GalNAc is part of the sugar chain on red blood cells that determines their blood type. This makes GalNAc critical for blood transfusions and immune recognition. Additionally, GalNAc aids in cell-cell recognition, helping cells identify each other during immune responses or tissue development. Some pathogens, like bacteria or viruses, bind to GalNAc to invade cells, making it a key target for infection research.
GlcNAc: The Multitasker
GlcNAc has a wider range of roles. It’s a major player in N-linked glycosylation, where sugars are attached to the nitrogen atom of asparagine amino acids in proteins. These sugar chains help proteins fold correctly, stay stable, and perform their jobs, such as in antibodies or cell surface proteins.
GlcNAc is also famous for forming chitin, a tough material made of long GlcNAc chains. Chitin builds the exoskeletons of insects and crustaceans, like crabs, and the cell walls of fungi. This structural role makes GlcNAc essential in nature. In humans, GlcNAc is a building block of cartilage components, which is why companies like NutriSynth Solutions use it in joint health supplements and OEM products.
Another key role is O-GlcNAc modification. Unlike O-linked glycosylation, this process adds a single GlcNAc to proteins inside cells, acting like a switch to regulate gene expression, metabolism, or stress responses. This is especially important in brain cells, where it supports functions like learning and memory.
Comparing Their Roles
Both sugars contribute to glycosylation, but they specialize differently: GalNAc in O-linked glycosylation and GlcNAc in N-linked glycosylation and chitin formation. GalNAc focuses on protective and recognition roles, like mucus or blood types, while GlcNAc is versatile, building structures and regulating cell signaling. Their C-4 configurations ensure they fit into specific enzymes, avoiding mix-ups in these complex processes.
Applications in Science and Medicine
The unique properties of GalNAc and GlcNAc make them valuable in science, medicine, and the supplement industry. Their applications range from advanced therapies to everyday health products.
GalNAc: Targeting the Liver and Beyond
GalNAc shines in liver-targeted therapies. It binds strongly to the asialoglycoprotein receptor (ASGPR) on liver cells, making it a perfect tool for delivering drugs directly to the liver. In RNA interference (RNAi) therapies, small RNA molecules are attached to GalNAc to silence specific genes. Drugs like Patisiran and Givosiran use GalNAc to treat rare genetic diseases, such as hereditary transthyretin amyloidosis, by targeting the liver precisely. This reduces side effects and boosts effectiveness.
GalNAc is also important in cancer research. Cancer cells often show abnormal sugar patterns, including GalNAc-containing structures, which can serve as biomarkers to detect cancer or as targets for therapies. Additionally, GalNAc is explored in vaccine development, as some pathogens display GalNAc on their surfaces, allowing vaccines to mimic these sugars to train the immune system.
GlcNAc: From Biomaterials to Supplements
GlcNAc has diverse applications, especially in the supplement and biomaterial industries. In biomaterials, chitin and its derivative chitosan (made by removing the acetyl group from GlcNAc) are used in medical products like wound dressings, drug delivery systems, and tissue scaffolds. These materials are safe for the body and break down naturally, making them ideal for medical use.
In the supplement industry, GlcNAc is a star ingredient. It’s a building block of cartilage components like glycosaminoglycans, which support joint health. NutriSynth Solutions, for example, produces high-purity GlcNAc as a raw material for dietary supplements aimed at relieving joint pain, particularly for osteoarthritis. Their OEM services also help brands create finished supplement products, such as capsules or powders, to meet consumer demand for joint health solutions .
GlcNAc also has research applications. In neurodegenerative diseases, O-GlcNAc modification affects proteins like tau, which forms tangles in Alzheimer’s disease. Studying GlcNAc could lead to new treatments for brain disorders. In antibacterial research, GlcNAc’s role in bacterial cell walls makes it a target for developing new antibiotics.
Comparing Applications
GalNAc’s applications are specific, focusing on liver-targeted drugs and cancer, while GlcNAc’s are broader, covering biomaterials, supplements, and brain research. NutriSynth Solutions leverages GlcNAc’s versatility to produce raw materials and OEM products for the growing health supplement market. Both sugars show how small molecules can have big impacts, but their unique properties guide them to different fields.
Key Differences and Similarities
Here’s a quick summary of what makes GalNAc and GlcNAc similar and different:
Similarities
- Both are amino sugars with the same molecular formula (C₈H₁₅NO₆).
- Both have an acetylated amine group at C-2, making them key in glycosylation.
- Both are used to build sugar chains that modify proteins and lipids.
Differences
- Structure: GalNAc has an axial C-4 hydroxyl group (based on galactose), while GlcNAc has an equatorial one (based on glucose).
- Function: GalNAc drives O-linked glycosylation and blood group antigens, while GlcNAc supports N-linked glycosylation, chitin, and O-GlcNAc signaling.
- Applications: GalNAc excels in liver therapies, while GlcNAc is used in biomaterials, supplements by companies like NutriSynth Solutions, and neurological research.
A table can help visualize these differences:
| Feature | GalNAc | GlcNAc |
|---|---|---|
| Parent Sugar | Galactose | Glucose |
| C-4 Hydroxyl | Axial | Equatorial |
| Key Role | O-linked glycosylation, blood types | N-linked glycosylation, chitin, signaling |
| Applications | Liver drugs, cancer biomarkers | Biomaterials, joint supplements, brain research |
Conclusion
GalNAc and GlcNAc may be small molecules, but their impact is enormous. A single difference at C-4—the position of a hydroxyl group—leads to unique roles in protecting tissues, building structures, and regulating cell functions. GalNAc helps create mucus and target liver therapies, while GlcNAc forms chitin, supports joints, and aids brain health. Companies like NutriSynth Solutions harness GlcNAc’s benefits to produce dietary supplements and OEM products, meeting the growing demand for health solutions.
These sugars show how tiny details in nature can lead to big breakthroughs. To dive deeper, check out the references below or explore glycobiology resources. GalNAc and GlcNAc are proof that even the smallest molecules can shape our health and future.
What is the difference between Gal and GalNAc?
Gal (galactose) is a simple sugar, while GalNAc (N-Acetylgalactosamine) is a modified version of galactose with an acetylated amine group (-NHCOCH₃) added at the second carbon. This change makes GalNAc an amino sugar with specific roles, like starting O-linked glycosylation, while Gal is a basic building block in many sugar chains.
What is the meaning of GlcNAc?
GlcNAc stands for N-Acetylglucosamine, an amino sugar derived from glucose with an acetylated amine group (-NHCOCH₃) at the second carbon. It’s a key molecule in glycosylation, chitin formation, and cell signaling processes.
What is the role of N-acetylglucosamine (GlcNAc) in cell signaling?
GlcNAc plays a vital role in cell signaling through O-GlcNAc modification, where a single GlcNAc is added to proteins inside cells. This acts like a switch, regulating processes like gene expression, metabolism, and stress responses, especially in brain cells for learning and memory.
What does O-GlcNAc do?
O-GlcNAc is a process that adds a single GlcNAc molecule to proteins inside cells. It controls protein function by turning on or off activities like signaling, metabolism, and stress responses, and it’s linked to brain health and diseases like Alzheimer’s when disrupted.
What is GalNAc used for?
GalNAc is used in liver-targeted drug delivery (e.g., RNAi therapies like Patisiran), cancer biomarker detection, and vaccine development. Its ability to bind liver cell receptors makes it valuable for precise medical treatments.
Is Gal GalNAc a biomarker of colon carcinogenesis?
Yes, Gal GalNAc (a structure involving GalNAc) can act as a biomarker for colon carcinogenesis. Abnormal sugar patterns, including GalNAc, on cancer cells are studied to detect and monitor colon cancer progression.
Is GlcNAc a sugar?
Yes, GlcNAc is a sugar. It’s an amino sugar derived from glucose, with an acetylated amine group, and it serves as a building block in glycosylation, chitin, and joint health supplements.
What is the GlcNAc in bacteria?
In bacteria, GlcNAc is a key component of the cell wall’s peptidoglycan layer, a mesh of sugar and protein that provides structure and protection. It’s linked with other sugars in a beta-1,4 pattern.
Is GlcNAc a glycan?
GlcNAc itself is not a glycan but a single sugar unit (monosaccharide). However, it’s a building block of glycans, which are complex sugar chains formed by linking GlcNAc with other sugars in processes like glycosylation.
Is N-acetylglucosamine linked to asparagine?
Yes, GlcNAc is the first sugar linked to asparagine in N-linked glycosylation, a process that attaches sugar chains to proteins, helping them fold and function correctly.
What does UDP-GlcNAc do?
UDP-GlcNAc (Uridine Diphosphate N-Acetylglucosamine) is a high-energy form of GlcNAc that cells use to donate GlcNAc during glycosylation and O-GlcNAc modification, driving the synthesis of sugar chains and protein regulation.
What is the mechanism of O-GlcNAc?
The O-GlcNAc mechanism involves two enzymes: O-GlcNAc transferase (OGT) adds GlcNAc to serine or threonine on proteins, and O-GlcNAcase (OGA) removes it. This dynamic addition and removal acts like a molecular switch for protein activity.
What does Gal GalNAc stand for?
Gal GalNAc refers to a sugar structure where galactose (Gal) is linked to N-Acetylgalactosamine (GalNAc), often found in O-linked glycosylation or as part of blood group antigens and cancer-related sugar patterns.
What is the primary difference between O-linked and N-linked glycosylation?
The primary difference is the attachment point: O-linked glycosylation attaches sugars (starting with GalNAc) to the oxygen of serine or threonine, while N-linked glycosylation attaches sugars (starting with GlcNAc) to the nitrogen of asparagine, affecting protein roles differently.
What is the difference between N-acetylglucosamine and N-acetyl-D-glucosamine?
N-acetylglucosamine and N-acetyl-D-glucosamine are the same compound. The “D” specifies the stereochemistry (D-configuration) of the glucose base, which is the standard form used in biology, so the terms are often interchangeable.
What is the difference between alpha-galactosidase and lipase?
Alpha-galactosidase is an enzyme that breaks down galactose-containing sugars (like in digestion), while lipase is an enzyme that breaks down fats. They target different molecules: sugars versus lipids.
References
- National Center for Biotechnology Information. (2025). PubChem Compound Summary for CID 439174, N-Acetylgalactosamine. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/N-Acetylgalactosamine
- National Center for Biotechnology Information. (2025). PubChem Compound Summary for CID 439213, N-Acetylglucosamine. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/N-Acetylglucosamine
- Varki, A., et al. (2017). Essentials of Glycobiology (3rd ed.). Cold Spring Harbor Laboratory Press. Available at https://www.ncbi.nlm.nih.gov/books/NBK310274/
- Alnylam Pharmaceuticals. (2025). Patisiran and Givosiran: GalNAc-Conjugated RNAi Therapeutics. Retrieved from https://www.alnylam.com/our-science/therapeutic-platforms
