
Glycine manufacturer
As a leading Creatine Monohydrate manufacturer, Gensei provides high-quality creatine monohydrate powder bulk for various applications. We are a trusted Creatine Monohydrate supplier with a strong presence in Creatine Monohydrate china, offering competitive pricing and reliable sourcing. Our state-of-the-art manufacturing processes ensure the purity and efficacy of our creatine monohydrate powder bulk. Choose Gensei as your preferred Creatine Monohydrate manufacturer and supplier for all your bulk creatine needs from China. We are dedicated to providing top-tier products and excellent service.
Please note: We are a wholesale supplier and have minimum order quantities.
Have questions about this product? Our team is here to help. For inquiries about multiple ingredients, please use the Contact Us option and include the list of ingredients in your message.
Glycine CAS No.: 56-40-6
Chemical Name: Aminoacetic acid
Synonyms:
- Gly
- Aminoethanoic acid
- Glycocoll
- Glycosthene
- Acdrile
CB Number: CB2285882
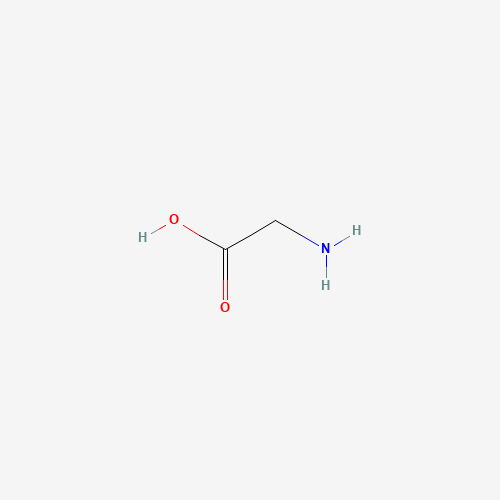
Molecular Formula: C₂H₅NO₂
Molecular Weight: 75.07 g/mol
MDL Number: MFCD00002580
Glycine Manufacturing Process Flowchart (Strecker Synthesis)
The primary industrial method for producing glycine is the Strecker amino acid synthesis. This flowchart outlines the key steps involved.
Formaldehyde (HCHO)
Ammonia (NH₃)
Reaction with Formaldehyde and Ammonia
(Formation of Aminoacetonitrile)
Hydrogen Cyanide (HCN)
Reaction with Hydrogen Cyanide
(Addition to Imino Group)
Conversion of Nitrile Group to Carboxylic Acid
(Using Acid or Base Catalyst)
Removal of Impurities
(Decolorization with Activated Carbon,
Ion Exchange Chromatography,
Removal of Salts)
Formation of Glycine Crystals
(Controlled Cooling and Concentration)
Centrifugation or Filtration
(To Isolate Glycine Crystals)
Removal of Moisture
(Using Techniques like Spray Drying or Fluid Bed Drying)
Testing for Purity, Heavy Metals,
Residual Solvents, and Other Specifications
Into Bags, Drums, or Other Suitable Containers
This flowchart provides a detailed overview of the industrial process for manufacturing Glycine using the Strecker Synthesis. Each step is carefully controlled to ensure the production of high-quality Glycine.
how is glycine manufactured?
Let’s go into a more detailed explanation of how glycine is manufactured, primarily focusing on the Strecker synthesis, which is the dominant industrial method:
The Strecker Amino Acid Synthesis: The Primary Route
The Strecker synthesis is a well-established chemical reaction used for the production of amino acids, and it’s the most economically viable method for manufacturing glycine on a large scale. The process essentially involves the reaction of an aldehyde (in this case, formaldehyde for glycine) with ammonia and hydrogen cyanide, followed by hydrolysis of the resulting nitrile.
Step 1: Formation of Aminoacetonitrile
This stage involves a two-part reaction.
Reaction with Formaldehyde and Ammonia: Formaldehyde (HCHO) first reacts with ammonia (NH₃) or an ammonium salt (like ammonium chloride, NH₄Cl) in an aqueous solution. This initial reaction forms an unstable intermediate, often considered to be an imine or a hemiaminal.
The simplified representation can be seen as:
HCHO + NH₃ → CH₂(NH) + H₂O (Imine formation)
or
HCHO + NH₃ ⇌ HOCH₂NH₂ (Hemiaminal formation)
Addition of Hydrogen Cyanide: Subsequently, hydrogen cyanide (HCN) is added to the reaction mixture. The cyanide ion (CN⁻) attacks the electrophilic carbon atom of the imine or reacts with the hemiaminal, leading to the formation of aminoacetonitrile (also known as glycinonitrile).
CH₂(NH) + HCN → NH₂CH₂CN
or
HOCH₂NH₂ + HCN → NH₂CH₂CN + H₂O
This aminoacetonitrile is a key intermediate in the synthesis of glycine.
Step 2: Hydrolysis to Glycine
The aminoacetonitrile then undergoes hydrolysis, which means it reacts with water to break the carbon-nitrogen triple bond of the nitrile group (-CN) and convert it into a carboxylic acid group (-COOH). This reaction yields glycine.
Catalyst: The hydrolysis is typically carried out in the presence of a strong acid (like hydrochloric acid, HCl) or a strong base (like sodium hydroxide, NaOH) as a catalyst to speed up the reaction.
Conditions: The reaction mixture is heated to a specific temperature for a certain period to ensure complete hydrolysis. The exact conditions (temperature, time, concentration of catalyst) depend on the specific process parameters employed by the manufacturer.
Using acid hydrolysis as an example:
NH₂CH₂CN + 2 H₂O + HCl → NH₃⁺Cl⁻CH₂COOH
The resulting glycine is in its hydrochloride salt form. To obtain free glycine, this solution needs to be neutralized.
Using base hydrolysis as an example:
NH₂CH₂CN + H₂O + NaOH → NH₂CH₂COONa + NH₃
This yields the sodium salt of glycine, which then needs to be acidified to obtain free glycine.
Purification Process (Detailed):
After the hydrolysis step, the glycine solution contains various impurities, including unreacted starting materials, byproducts, salts, and colored compounds. A series of purification steps are crucial to obtain high-purity glycine suitable for its intended applications (e.g., food, pharmaceuticals).
- Decolorization: Activated carbon is often added to the glycine solution. The porous structure of activated carbon allows it to adsorb colored impurities and other organic contaminants. After a sufficient contact time, the activated carbon is removed by filtration, resulting in a clearer solution.
- Ion Exchange Chromatography: This technique is highly effective in removing ionic impurities, including residual salts and any charged byproducts. The glycine solution is passed through columns packed with ion exchange resins. These resins selectively bind ions based on their charge, allowing the neutral glycine molecules to pass through. Different types of resins (cationic and anionic) can be used in sequence to remove a wide range of ionic contaminants.
- Removal of Salts: Depending on the hydrolysis method and the subsequent neutralization steps, the glycine solution might contain inorganic salts. Techniques like membrane filtration (e.g., reverse osmosis or nanofiltration) can be used to separate glycine from these dissolved salts. Further crystallization steps can also aid in removing salts as glycine crystals precipitate out, leaving the more soluble salts in the solution.
Crystallization Process (Detailed):
Crystallization is a critical step for obtaining glycine in a solid, pure form.
- Concentration: The purified glycine solution is concentrated, typically by evaporating excess water under controlled conditions (often reduced pressure and elevated temperature to increase efficiency and prevent degradation). The goal is to reach a supersaturated state where the concentration of glycine exceeds its solubility at a given temperature.
- Cooling and Seed Crystallization: The concentrated solution is then carefully cooled. As the temperature decreases, the solubility of glycine further decreases, promoting crystallization. To control the crystal size and uniformity, small seed crystals of glycine are often added to the solution. These seed crystals provide nucleation sites for further glycine molecules to attach and grow, leading to the formation of larger, more easily filterable crystals.
- Crystal Harvesting: Once the glycine crystals have reached the desired size, they are separated from the remaining solution (mother liquor) using techniques like centrifugation or filtration. Centrifugation uses centrifugal force to separate the solid crystals from the liquid, while filtration involves passing the mixture through a filter medium that retains the solid crystals.
Drying:
The separated glycine crystals still contain moisture and need to be dried to obtain a stable, free-flowing powder with a low moisture content, which is essential for storage and handling. Common drying methods include:
- Spray Drying: The glycine solution is sprayed as a fine mist into a hot gas stream. The water evaporates rapidly, leaving behind fine particles of dry glycine powder. This method is often used for large-scale production and results in a powder with good flowability.
- Fluid Bed Drying: The wet glycine crystals are placed in a fluidized bed where hot air is blown through them. The air suspends the particles and provides efficient heat transfer for drying.
- Vacuum Drying: Drying under reduced pressure lowers the boiling point of water, allowing for drying at lower temperatures, which can be beneficial for maintaining the quality of the glycine.
Quality Control:
Throughout the entire manufacturing process, rigorous quality control measures are implemented to ensure the final1 glycine product meets the required specifications for purity, assay (percentage of glycine), moisture content, heavy metals, residual solvents, and other parameters. Techniques like High-Performance Liquid Chromatography (HPLC), gas chromatography (GC), and atomic absorption spectroscopy (AAS) are commonly used for these analyses.
Alternative Manufacturing Methods (Briefly):
While the Strecker synthesis is dominant, other methods exist but are less frequently used for large-scale production:
- Hydrolysis of Proteins: Proteins rich in glycine, such as gelatin, can be hydrolyzed using acids or bases to release glycine. However, this method yields a mixture of amino acids, and separating glycine from this mixture can be complex and costly.
- Fermentation: Certain microorganisms can be genetically engineered to produce glycine from simple carbon and nitrogen sources. This method is gaining interest as a potentially more sustainable and environmentally friendly alternative, but it is not yet as widely used as the Strecker synthesis for bulk production.
Conclusion:
The manufacturing of glycine primarily relies on the Strecker synthesis, a well-defined chemical process involving the reaction of formaldehyde, ammonia, and hydrogen cyanide followed by hydrolysis. The subsequent purification and crystallization steps are crucial for removing impurities and obtaining high-quality glycine in a stable, powdered form. Each stage of the process is carefully controlled and monitored to ensure the final product meets the stringent requirements for its various applications.
Our State-of-the-Art Manufacturing Facilities
Explore our modern facilities equipped with advanced technology to ensure the highest quality in the production of your vitamins, herbal extracts, minerals, and amino acids.











FAQs
Some studies suggest that glycine may actually help lower blood sugar levels or improve insulin sensitivity in individuals with type 2 diabetes. However, more research is needed, and people with diabetes should consult their doctor before using glycine supplements.
No, glycine is a molecule, specifically an amino acid. While the glycine molecule is electrically neutral at physiological pH (existing as a zwitterion), it is composed of multiple atoms (carbon, hydrogen, nitrogen, and oxygen).
No, glycine is generally considered hydrophilic. While its side chain is just a hydrogen atom (the simplest and non-bulky), the presence of the polar amino (-NH₂) and carboxyl (-COOH) groups makes the molecule overall polar and capable of interacting favorably with water.
Yes, glycine acts as an inhibitory neurotransmitter in the central nervous system, particularly in the spinal cord, brainstem, and retina. It plays a crucial role in regulating motor and sensory functions.
Yes, glycine is a major component of collagen, making up approximately one-third of its amino acid composition. The unique structure of glycine allows for the tight triple helix formation characteristic of collagen.
Glycine is hydrophilic.
In the central nervous system, glycine is primarily inhibitory. It binds to specific receptors, leading to the influx of chloride ions into the neuron, which hyperpolarizes the cell and makes it less likely to fire an action potential.
It is strongly recommended to consult with your doctor before taking glycine and Ativan (lorazepam) together for sleep. Both substances can have sedative effects, and combining them could potentially lead to excessive drowsiness, respiratory depression, or other adverse effects. Your doctor can assess your individual situation and advise on the safety and appropriateness of this combination.
Yes, glycine is the only achiral amino acid. Chirality in amino acids arises from the central carbon atom being bonded to four different groups. In glycine, the central carbon is bonded to an amino group, a carboxyl group, a hydrogen atom, and another hydrogen atom, making two of the groups identical.
No, glycine is considered polar. The presence of the amino and carboxyl groups, which can form hydrogen bonds and carry partial charges, makes the glycine molecule polar overall.
Glycine is polar.
- Animal Feed: As a component in animal feed to improve growth and nutrition.
- Metal Complexing: In various industrial processes for its ability to form complexes with metal ions.
- Chemical Synthesis: As an intermediate in the synthesis of other chemicals, including pharmaceuticals and agrochemicals.
- Buffering Agent: In some industrial applications where pH control is important.
- Electroplating: As an additive in electroplating solutions.
There is no strong evidence suggesting negative interactions between glycine and N-acetylcysteine (NAC). Some research even suggests potential synergistic benefits for antioxidant and liver support. However, it's always best to consult with a healthcare professional before combining supplements, especially if you have any underlying health conditions or are taking other medications.
In the central nervous system, glycine is primarily inhibitory.
Some preliminary research suggests that glycine supplementation may have a modest blood pressure-lowering effect in some individuals. However, more research is needed to confirm these findings and determine the optimal dosage and long-term effects. Glycine should not be considered a replacement for conventional blood pressure management strategies.
Similar to question 8, it is strongly recommended to consult with your doctor before taking glycine with Ativan (lorazepam) due to the potential for additive sedative effects.
Yes, glycine is highly soluble in water due to its polar nature and its ability to form hydrogen bonds with water molecules.
Industrial grade glycine refers to glycine that is manufactured for industrial applications and may have different purity specifications compared to glycine intended for food or pharmaceutical use. While it is still primarily glycine, the acceptable levels of certain impurities might be higher.
Glycine is an amino acid and contains a small number of calories (approximately 4 calories per gram). For individuals following a strict metabolic fast or a zero-calorie fast, consuming glycine would technically break the fast. However, for those fasting for general health benefits or caloric restriction, the small caloric content of a typical glycine dose (e.g., 1-3 grams) might not be considered significant enough to break the fast by some protocols. The specific rules of the fast being followed should be considered.
Current scientific evidence does not strongly support a direct link between glycine supplementation and increased testosterone levels in humans. While some studies in animals have shown potential effects on hormone levels, these findings have not been consistently replicated in human research.
It is strongly recommended to consult with your doctor before taking Ativan (lorazepam), glycine, and magnesium together. All three substances can have calming or sedative effects, and combining them could significantly enhance these effects, potentially leading to excessive drowsiness, confusion, or other adverse reactions. Your doctor can assess your individual health status and advise on the safety of this combination.
Yes, you can generally take glycine and magnesium glycinate together. Magnesium glycinate is a form of magnesium where the magnesium ion is bound to glycine molecules. Taking additional glycine alongside it would essentially increase your overall glycine intake. While generally considered safe for most people, it's always wise to consult with a healthcare professional if you have any underlying health conditions or concerns about potential interactions.
This question refers to a brand of watches called "Glycine," which is unrelated to the amino acid glycine. As an AI, I do not have personal opinions on the quality of specific watch brands. You may want to consult watch reviews or forums for opinions on Glycine watches.

